I’d like to share further insights gleaned from clinical practice, informed by the evidence – and offer a bit of paradigm shift in the way we approach and manage tendinopathies. Here is a précis of my presentation at the recent Canberra APA Research Symposium.
Clinical significance:
Tendinopathies continue to be an increasingly common clinical presentation and may account for around 30% of musculoskeletal complaints presenting to the general medical practitioner. [i] They are notoriously difficult and frustrating to treat – and recurrence is common. [ii] Despite an increasing body of science, their aetiology remains obscure.
Background:
Tendinopathies are considered to be an ‘over-use’ or loading problem – yet their incidence is not necessarily related to excessive physical activity.[iii] Identification of the potential intrinsic ‘risk factors’ for the development of tendinopathy is limited, [ii] [iv] [v] and imaging abnormalities do not necessarily correlate with symptoms. [vi] [vii] Diagnosis is primarily based on the clients history and physical examination in the clinic. Misdiagnosis is common. [ii]
Much of the research to date has focused upon the structural pathology of the tendon and local mechano-biological mechanisms, [viii] and this has dictated many current treatment approaches. [ix] [x]
More recent research has begun to look at neuroscience for possible contributors outside the tendon, including altered peripheral and central mechanisms of pain modulation. [xi] [xii] [xiii] [xiv] If there is persistent chronic nociception from the periphery, CNS processing is altered – and this may lead to ‘central sensitisation’ [xv] of the nervous system: “creating pain sensitivity – with less provocation”
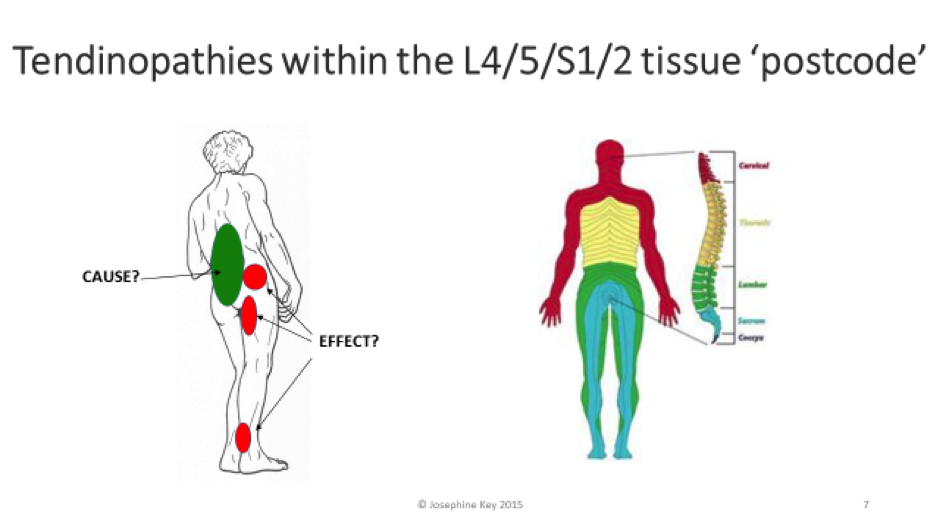
Could the problem lies somewhere in between the tendon in the periphery and the CNS?
My proposed Hypothesis:
- Spinal dysfunction is ‘the’ underlying driver of most tendinopathies – it is the adverse spinal loading and ‘overuse’ (which occurs in typical patterns) – which in turn goes on to affect peripheral function. Tendinopathies – (and other diverse symptoms) can result.
- So, peripheral tendinopathies can be seen as a reflection of a wider ‘functional pathology of the movement system” [xvi] – involving interlocking dysfunctions between the myo-fascial, articular and neural systems
- HOW?: – Altered spinal loading and movement patterns lead to adverse ‘segmental bother’, and the potential for ‘sub-clinical’ or actual neural irritation and pain and disturbed function in various tissues throughout the segment’s ‘innervation postcode.
- Treatment approaches which address the more proximal spino-pelvic dysfunction via tailored manual and exercise therapy can significantly alter the peripheral pain and tissue dysfunction – which then become more amenable to direct treatment and loading – yielding better outcomes in a shorter time frame.
Defence of this hypothesis:
- The potential role of the spine in the aetiology and patho-physiology of tendinopathy has been little considered to date.
- This is strange as the spine is ‘the’ fundamental support involved in all movement!
- Its control is complex and not well understood … and
- Its health is vulnerable to changes in motor behaviour.
- Spinal pain research has demonstrated deficient patterns of deep muscle system and ’core’ control [xvii] [xviii] and associated hyperactivity of the superficial trunk muscles. [xix] [xx]
The spinal segmental level of the nervous system is an important relay station between the PNS and the CNS
- The spine houses a significant part of the CNS – yet its control is supported by the PNS – the spine itself its part of ‘the periphery’!
- Janda 33 years ago told us of the significance of dysfunction at this level of the nervous system – which is commonly overlooked.[xvi]
- It is important to recognise that spinal segmental dysfunction can be a potent source of chronic ‘peripheral nociceptive input’ to the CNS,
- It can also further generate the peripheral changes which are part of the tendinopathy picture and lead to the functional changes in the nervous system associated with tendinopathy.
- This can be further reflected by other changes in the CNS such as altered reflex behaviour and movement patterns, fatigue etc. [xvi]
Tendinopathy research has implicated the spine and segmental dysfunction:
- In 1998 Maffulli pondered a possible correlation between achilles tendon rupture and spinal involvement – 60% of the cohort had suffered chronic LBP/Sciatica. [xxiii]
- Bilateral achilles tendinopathy is not unusual (41%) -”Unilateral surgical treatment for patients with mid-portion Achilles tendinopathy may result in bilateral recovery” – indicating a potential role of CNS in generating tendon pain: [xxiv]
- Developing symptoms of tendinopathy on the other side is common:
- More recent studies illustrate CNS, spinal segmental and peripheral nervous system involvement in the pathophysiology of tendinopathy.
- Evidence of increased spinal cord hyper-excitability found in lateral epicondylalgia – “continued peripheral afferent stimulation results in facilitation of nociceptive pathways – “altered pain signalling is likely to be the underling mechanism in lateral epicondylalgia” [xxvii]
However, is the conclusion drawn by the researchers cause or effect? – of course altered peripheral afference will affect the CNS – but… is the cause of the peripheral problem spinal dysfunction? It’s a vicious cycle!
- Increased corticospinal excitability has been found in patellar tendinopathy – and this is positively related to symptom duration. [xxvii]
- Isometric quadriceps contractions at 60° knee flexion significantly reduced patellar tendinopathy pain immediately during single leg decline squat for 45’ – and this was also paralleled by a reduction in cortical inhibition, and increased MVC quads strength. [xxix]
The findings of the bilateral, segmental & CNS changes found in unilateral tendinopathy implicate spinal dysfunction
- Widespread mechanical pain hypersensitivity has been shown in unilateral epicondylalgia – pressure pain thresholds (PPTs) were significantly reduced bilaterally. [xxx] They chose to include the C5/6 vertebral level “by its segmental relationship to the lateral epicondyle” – They found: “A significant decrease in PPTs bilaterally over the lateral epicondyle and C5/6 Zygapophyseal Joint may represent the existence of segmental sensitisation of the nociceptive system in lateral epicondylalgia” – and “indicates a broad involvement of CNS in lateral epicondylalgia”
- A bilateral reduction in gastrocnemius EMG amplitudes has been shown in runners with unilateral achilles tendinopathy. [xxxi]
- Increased lower limb stiffness and delayed muscle activity found in Achilles Tendinopathy during a hopping task. [xxxii]
There are numerous potential mechanisms for the SNS to contribute to tendon pain/disease – whether through vasoconstriction, neurogenic inflammation or direct effects on sensory nerves [xxxiii]:
- The SNS can respond bilaterally to unilateral stimuli [xiv] (this may help explain why 41% people with achilles tendon pain later developed pain on hitherto painless other side).
- Unilateral lateral epicondylalgia cohort all demonstrated bilateral widespread mechanical hyperalgesia. [xxxiv] Thermal hyperalgesia distinguished those with severe pain.
- The researchers concluded: “Findings may implicate a combination of central, peripheral and sympathetic nervous system dysfunction”
- Painful paratendinous tissues have shown increased SNS innervation – this suggests para-tendinous tissue may play more of a role in achilles tendinopathy than the tendon itself [xxxiii].There is increasing interest in the potential role of upregulation of the Sympathetic Nervous System (SNS) in tendinopathy
A systematic review on the potential role of the SNS and tendinopathy, (Jewson 2015) points out that global in vivo SNS activity has not been investigated in the context of tendinopathy
Yet spinal dysfunction is a prime source of sympathetic upregulation!
Role of fascial dysfunction in tendinopathy
- Fascia research is a rapidly evolving field.
- Fascial network – is a tensegrity structure for transferring load, dissipating force and spring loading for movement efficiency – tendons are part of fascial network!.
- Increased basal tension and lack of ‘slide’ in the fascial system diminishes force generation, mechano-sensory fine tuning and can be a potent source of nociception due to the predominance of ‘wide dynamic range’ neurons [xxxv] – especially in inflammation[xxxvi].
- Pain over the tendon may be caused by more proximal fascial dysfunction [xxxvii] [xxxviii]– There is increasing interest in the role of the paratendinous tissues [xxxix]. Increased para-tendon thickness has been shown in in achilles tendinopathy [xl] by US imaging.
- Clinically spinal dysfunction is linked to dysfunction through the lower limb fascial system.
So…. which factors point to the probable role of spinal dysfunction in lower limb tendinopathies?
Clinical evidence identifies a number of qualitative, readily identifiable, intrinsic risk factors associated with lower limb tendinopathies
Essentially this looks at HOW the individual’s axial movement system FUNCTIONS!
Relevant in the subjective history:
- Low back discomfort: ‘mild’, ‘pain’ and/or stiffness is chronic: ‘background music’
- History of past ‘injuries’, recurrent injuries/tendinopathies/ bursitis etc. – and /or multiple ‘injury’ or pain sites
- Gym attendance!! – BIG dysfunction factories! (and often Pilates – and sometimes yoga). The gym philosophy is ‘all about strength’ rather than control. On the whole there is poor exercise prescription – with little focus on the deep muscle system. Instead, most protocols activate the more superficial global muscle system – pretty much all with effort – and breath-holding! These global dominant movement patterns are learned – and become indelibly imprinted in CNS.
Yet the research is clearly telling up that people with back pain disorders have an underactive deep system and propensity to overactive global superficial muscle activity!
- Needing to stretch all the time – many commonly also use ‘the roller’ for release
- Usually also Apparent:
- Sedentary worker
- Stress in ADL – affects breathing patterns; upregulates sympathetic NS drive
- Observe: physical characteristics that predispose an athlete to lower limb tendinopathies – risk factors if you like!:
- Altered standing postural alignment
- Presence of ‘tell-tale signs’ over the posterior torso: Central Posterior Cinch [xli]; muscle contours including a ‘Key Sign’ etc.
- Postural collapse in sitting – is universal!
- Changed movement kinematics e.g. Forward bend pattern; squat
Testing and observation
be alert to the common intrinsic physical risk factors that feed into the tendinopathy picture:
Inability to assume a naturally antigravity ‘neutral spine’ in sitting
Attempts at ‘sitting up’ or achieving a ‘neutral spine’ involve poor pelvic control and ‘lock-in’ strategies via excess superficial neuromuscular activity higher up spine (CPC)
Dysfunctional breathing patterns: upper chest breathing; breath holding in movement
Poor patterns of ‘core control’ and spinal stabilisation from the deep muscle system
Poor patterns of pelvic control – loss of control of lumbar lordosis and intrapelvic movement. This causes the joints and soft tissues to be repetitively loaded into end range flexion & sacral counter-nutation. The L4/5/S1/2 segments are most affected & functional changes are likely within the innervation field of the lower limb. Compensatory CPC higher up drives autonomic nervous system dysfunction
Inability to control spinal alignment with lower limb stretches – further bother spine which becomes the victim
On review: the client is invariably doing an exercise programme/gym routine which further compromises healthy spinal functional control and bother the spine
Increased muscle tone around spine is readily apparent ‘at rest’ when recumbent – and this is associated with fascial dysfunction – “Where there is smoke there is fire”!
Treatment
The spine is tricky to treat!! High level abilities for observation and palpation is required
- Careful considered palpation of the spinal tissues & specific testing of ‘joint play’ will confirm or otherwise the likelihood of a ‘segmental driver’ input to the tendinopathy
- When positive, local stiffness, tension & reactivity in relevant joints & related soft tissues is found – & probably acute local allodynia & autonomic changes.
- However, it is important to appreciate that provocation of peripheral pain referral is rare.
- Increased resting tone and fascial dysfunction may well be apparent in the relevant ‘innervation postcode’?
Implications
Understanding the wide reach of spinal dysfunction and managing tendinopathies as part of a more regional and global movement dysfunction may improve outcomes.
The Key Approach Model for tendinopathies, as outlined, sheds light on the aetiology of lower limb tendinopathies by outlining the intrinsic risk factors which may predispose to soft tissue break down. It also has implications for exercise prescription and prevention. And offers scope for future research.
References:
[i] Forde MS et al 2005 Prevalence of musculoskeletal disorders in union ironworkers J Occup Environ Hyg 2(4):203-12
[ii] Scott et al 2013 Sports and exercise-related tendinopathies: a review of selected topical issues by participants of the second International Scientific Tendinopathy Symposium (ISTS) Vancouver 2012. BJSM published online April 12 2013
[iii] De Jonge S et al 2011. Incidence of midportion Achilles tendinopathy in the general population. British J Sports Medicine October
[iv] Maffulli N et al 2003. Types and epidemiology of tendinopathy Clinics in Sports Medicine 22:675-692
[v] Gaida JE and Cook J 2008. Risk factors for overuse tendinopathy. Australasian Musculoskeletal Medicine November
[vi] Cook J et al 1998 Patellar tendon ultrasonography in asymptomatic active athletes reveals hypoechoic regions: a study of 320 tendons. Victorian Institute of sport tendon study group Clin J Sports Med 8:73-77
[vii] Frost et al 1999. Is supraspinatus pathology as defined by MRI associated with clinical signs of shoulder impingement? J Shoulder Elbow Surg 8:565-8
[viii] Xu and Murrell 2008. The basic science of tendinopathy Clin Orthop and Related Research July 466(7)1528-1538
[ix] Cook and Purdham 2009. Is tendon pathology a continuum? A Pathology model to explain the clinical presentation of load-induced tendinopathy Br. J Sports Med June 43(6):409-16
[x] Grimaldi et al 2015. Gluteal tendinopathy: A review of mechanisms, assessment and management. Sports Med August 45(8):1107-19
[xi] Rio et al 2014. The pain of tendinopathy: Physiological or pathophysiological? Sports Med. Jan.44(1): 9-23
[xii] Lim et al 2012. Evidence of spinal cord hyper-excitability as measured with nociceptive flexion reflex (NFR) threshold in chronic lateral epicondylalgia with or without a positive neurodynamic test. J Pain; 13:676–84.
[xiii] Ackerman et al 2014 Neuronal pathways in tendon healing and tendinopathy – update. Front Biosci (Landmark edition) Jun 1 (19):1251-78
[xiv] Andersson et al 2011. Tenocyte hyper-cellularity and vascular proliferation in a rabbit model of tendinopathy: contralateral effects suggest the involvement of central neuronal mechanisms. Br J Sports Med 45:399–406.
[xv] Woolf C 2010. Central sensitisation: implications for the diagnosis and treatment of pain
[xvi] Janda V 1982 Introduction to functional pathology of the motor system VII Commonwealth and International Conference on Sport, Physical Education, Recreation and Dance Proc. Vol 3
[xvii] Hodges PW, Richardson CA 1996. Inefficient muscular stabilisation of the lumbar spine associated with low back pain: a motor control evaluation of transversus abdominis. Spine 21, 2640–2650.
[xviii] Hodges, P.W., Moseley, G.L., et al 2003. Experimental muscle pain changes feedforward postural responses of the trunk muscles. Experimental Brain Research 151, 262–271
[xix] O’Sullivan PB et al. 2002 Altered motor control strategies in subjects with sacroiliac joint pain during active straight leg raise test. Spine, 27(1):E1-E8
[xx] Belavey DL et al 2007. Tonic to phasic shift of lumbo-pelvic muscle activity during 8 weeks of bedrest and 6 months follow-up J Applied Physiology 103:48-54
[xxi] Bogduk N Twomey L 1987 Clinical anatomy of the lumbar spine Churchill Livingstone p.98
[xxii] Grieve G 1981 Common vertebral joint problems
[xxiii] Maffulli et al. 1998 Achilles tendon rupture and sciatica: a possible correlation. Br J Sports Med 1998;32:174–7
[xxiv] Alfredson et al 2012 Unilateral surgical treatment for patients with mid-portion Achilles tendinopathy may result in bilateral recovery Br J Sports Med Published Online First: 28 November 2012 doi:10.1136/bjsports-2012-091399
[xxv] Paavola M et al 2000 Long term prognosis of patients with Achilles tendinopathy. An observational 8 year follow-up study Am J Sports Med 28(5):634-42
[xxvi] Aroen et al 2004 Contralateral tendon rupture risk is increased in individuals with a previous Achilles tendon rupture Scand J Med Sci Sports 14(1):30-33
[xxvii] Lim et al 2012. Evidence of spinal cord hyper-excitability as measured with nociceptive flexion reflex (NFR) threshold in chronic lateral epicondylalgia with or without a positive neurodynamic test. J Pain; 13:676–84.
[xxviii] Rio et al 2014 Patellar Tendinopathy: Looking Outside the Tendon… Abstract Br J Sports Med 48::A57-A58
[xxix] Rio E Et al 2015 Isometric exercise induces analgesia and reduces inhibition in Patellar Tendinopathy. published online May Br J Sports Med 2015;0:1–8. doi:10.1136/bjsports-2014-094386
[xxx] Fernández-Carnero et al 2009.Widespread mechanical pain hypersensitivity as a sign of central sensitisation in unilateral epicondylalgia Clin J Pain Vol 25 (7):555-561
[xxxi] Baur H et al 2011 Comparison in lower leg neuromuscular activity between runners with unilateral mid-portion Achilles tendinopathy and healthy individuals. J Electromyography & Kinesiology 21(3):499-505
[xxxii] Debenham et al 2014.Achilles tendinopathy alters stretch shortening cycle behaviour during a sub-maximal hopping task. Br. J Sports Med 48:A19-A20
[xxxiii] Jewson J et al 2015. The sympathetic nervous system and tendinopathy: A systematic review. Sports Med Published online February
[xxxiv] Coombes et al 2012. Thermal hyperalgesia distinguishes those with severe pain and disability in unilateral hyperalgesia Clin J Pain 28(7):595-601
[xxxv] Klingler et al 2014Clinical relevance of fascial tissue and dysfunctions Curr Pain Headache Rep 18:439
[xxxvi] Hoheisel U et al 2015 Innervation changes induced by inflammation of the rat thoracolumbar fascia Neuroscience 300 May DOI: 10.1016/j.neuroscience.2015.05.034
[xxxvii] Stecco L 2004 Fascial Manipulation for musculoskeletal pain Piccin Padova Italy
[xxxviii] Schleip R (Ed) 2015. Fascia in sport and movement Handspring Publishing Edinburgh
[xxxix] Stecco C et al 2013 Histological study of the paratendinous tissues Surg Radiol Anat DOI 10.1007/s00276-013-1244-8
[xl] Stecco A et al 2014. Comparative ultrasonographic evaluation of the Achilles paratenon in symptomatic and asymptomatic subjects: an imaging study. Surg Radiol Anat Published online July 2014
[xli] Key J 2010. Back Pain: A movement problem. A clinical approach incorporating relevant research and practice. Elsevier, Edinburgh